720.341.0852 720.341.0852
|
 Send Us Email Send Us Email
|
|
|
|
|
|
|
|
|
|
|
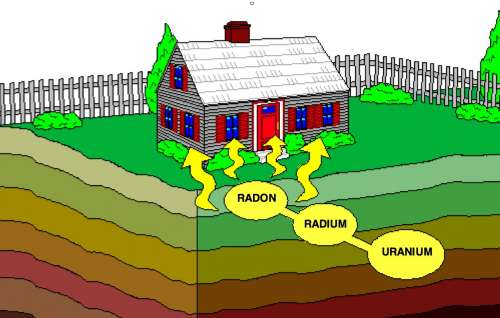
RadonWhat is Radon?Radon is a gas produced by the radioactive decay of the element radium. Radioactive decay is a natural, spontaneous process in which an atom of one element decays or breaks down to form another element by losing atomic particles (protons, neutrons or electrons). When solid radium decays to form radon gas, it loses two protons and two neutrons. These two protons and two neutrons are called an alpha particle, which is a type of radiation. The elements that produce radiation are referred to as radioactive. Radon itself is radioactive because it also decays, losing an alpha particle and forming the element polonium. What is Radioactivity?Radioactivity is the property of some atoms that causes them to give off energy spontaneously as particles or rays. Radioactive atoms emit ionizing radiation as they decay. Why Some Atoms Are Radioactive?
The balance of the forces in the nucleus of an atom determines whether a nucleus
is stable or unstable (radioactive).
• ejecting neutrons and protons It is this release of energy or particles that can have a detrimental effect on one's health. Decay ChainsMost naturally occurring radioactive materials and many fission products undergo radioactive decay through a series of transformations, rather than in a single step. Until the last step, these radionuclides emit energy or particles with each transformation and become another radionuclide. This series of decay, known as a decay chain, ends in a stable nuclide. In the case of radon gas (radon-222) it begins with Uranium (uranium-238) which is present in all soil. As it decays through a series of steps to become stable, it changes and becomes another radionuclide with each step. Radon too breaks down even further forming "Radon Decay Products", which are also radioactive. RDPs are different from actual radon in a few ways. The
characteristics of RDPs include:
These decay products capable of easily attaching themselves to solid objects such as dust, smoke, walls, floors, clothing, or any other objects. If the RDPs (Radon Decay Products) attach to surfaces, they are no longer floating in the air and are of little concern. If they attach to ducts or smoke particles, they can be carried into the lungs where they can cause lung cancer. Radon DangerWe measure radon in "picocurie per liter" (pCi/L); a unit of radioactivity corresponding to an average of one decay every 27 seconds in a volume of one liter of air. The EPA has set a recognized value of 4 pCi/L as the maximum average amout of radon gas before the need to use mitigation efforts in order to make the air quality safer. The EPA estimates that radon is responsible for about 20,000 lung cancer deaths each year in the United States. Exposure to radon is the second leading cause of lung cancer after smoking. Radon is an odorless, tasteless and invisible gas produced by the decay of naturally occurring uranium in soil and groundwater. Radon is a form of ionizing radiation and a proven carcinogen. Most of the epithelial cellular damage is not caused by breathing in radon gas itself, which is removed from the lungs during exhalation, but by radon's short-lived decay products (half-life measured in minutes or less). When inhaled, these decay products may be deposited in the airways of the lungs. The RDPs subsequently emit alpha particles as they decay further. The total amount of energy emitted by the progeny is several hundred times that produced in the initial decay of radon. The increased risk of lung cancer from radon results primarily from these alpha particles irradiating lung tissue. When an alpha particle passes through a cell's nucleus, the person's DNA is likely to be damaged. More specifically, available data indicate that alpha particle penetration of the cell's nucleus may cause genomic changes, most typically in the form of point mutations and transformations. How Does it Enter a Home?Radon as a gas is able to migrate through the soil in into a structure. There are four main factors that drive radon into homes. All of these factors exist in most homes throughout the country.
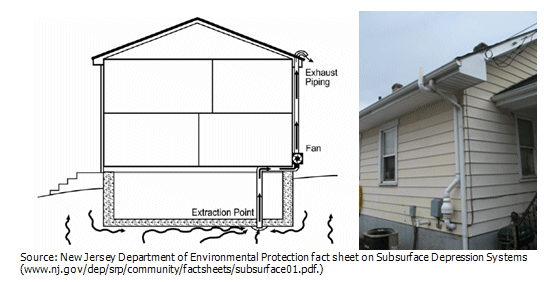
How To Test for RadonTesting for the levels of radon is actually very easy to do. A home owner can purchase a good test kit at the local hardware store for under $30.00. These tests typically take 2 or 3 days to perform and then another 5 to 10 days to get the results because of the fact that the test kit must be sent to a lab for analysis and the lab has to respond with the test data. In some instances this method works quite well, although in a real estate transaction you may not be afforded that much time to present your findings. The other method is to use a continuous radon monitoring system. Continuous radon monitoring equipment, although very expensive, will provide an immediate dataset of the radon quantities detected after the 48 hour test complete. These devices will sample the air typically on an hourly basis and based on the average quantity of radon detected over that period of time will provide enough information to make an informed decision concerning the need for remediation equipment to be installed without the delay of sending a test kit to a lab and waiting for a response. What Should I Do if Radon Levels Are High?First it is important to realize that radon is a very common issue, at the same time it should not be taken lightly. There are a number of ways to remediate radon and one of common method used in existing homes is to install an active sub-slab depressurization system as shown below. If these systems are new to you take notice as you drive or walk around various neighborhoods and you will find that they are common.
Where to Get More Information on Radon?Our brief discussion has only highlighted some of the main points with regard to radon. There are a number of fine resources to learn more on the subject. Below are just a few suggestions: |
| Homemall" href="about.htm">About Us Services Questions? Contact InterNACHI Articles |